Image CDC PHIL 10924

Version:1.0 StartHTML:0000000166 EndHTML:0000018057 StartFragment:0000002613 EndFragment:0000018021 SourceURL:file://localhost/Users/krohde/Desktop/Malaria.doc
Wellcome Trust Images


OVERVIEW
Agents and life cycle
Malaria (Italian “bad air”) is a disease caused by protistan (unicellular) parasites belonging to the Apicomplexa (sporozoans), genus Plasmodium. Many species are known which are specific to various reptiles, birds and mammals [1] [2] [3]. Human malaria is caused by four species, Plasmodium falciparum, P. vivax, P. malariae and (less commonly) P. ovale. The species P. knowlesi and P. cynomolgi, normally infecting macaque (and the second species also other) monkeys in Asia, rarely infect humans. The parasites have an indirect life cycle, i.e. they require two hosts. The intermediate host (humans), harbours sexually immature stages, the final host (in human malaria many mosquito species of the genus Anopheles) harbours sexually mature stages.
Mosquitoes inject malaria sporozoites into the bloodstream of humans when sucking blood. These stages penetrate into cells of the liver parenchyma, where they grow up to large cells, so-called megaschizonts (although some sporozoites of P.vivax and P.ovale may survive as “sleeping stages” (= hypnozoites) for long periods in the blood plasma before infecting host cells). After a number of days these stages multiply by asexual fission (= preerythrocytic or exoerythrocytic schizogony) giving rise to small merozoites, which are released into the bloodstream to infect erythrocytes(= red blood cells). They are now referred to as trophozoites (= feeding stages). They grow up to schizonts smaller than those in the liver, which again multiply by asexual fission (= erythrocytic schizogony), producing several to many (depending on the species) daughter cells, the merozoites. Host cells are destroyed during schizogony, their toxic substances released into the blood. Importantly, schizogony in the infected blood cells is synchronized, i.e. toxic substances from many destroyed cells are released at more or less the same time, leading to the malaria symptoms (chills, fever attacks, severe headache, etc.). Merozoites infect other erythrocytes and the process of asexual reproduction is repeated many times. At a later stage, some of the merozoites are not transformed into feeding stages and schizonts, but into sexual cells (= male and female gametocytes). These circulate in the blood, where they cannot develop further until ingested by an Anopheles-mosquito. In the digestive tract of the mosquito, male gametocytes give rise to several male gametes by a process called exflagellation (many flagella-like gametes arranged around a central residual body), whereas the female gametocyte is transformed into a single female gamete. One male gamete penetrates into a female one and fertilizes it forming a zygote which is mobile and therefore called an ookinete. It penetrates into the wall of the mosquito’e digestive tract and surrounds itself with a cyst wall, thus becoming an oocyst, containing a large number of sporozoites. After some time, the cyst wall of the oocyst raptures, releasing numerous sporozoites into the body cavity, from where they migrate mainly into the salivary glands of the mosquito. They enter the bloodstream of humans when they are injected by the mosquito.
The four major human species of malaria differ in morphology and symptoms produced, but in all species the first disease symptoms occur only once schizogonies in the blood have produced large numbers of merozoites infecting erythrocytes with subsequent breakup of large numbers of blood cells leading to the release of large quantities of toxic substances. In other words, no symptoms can be expected as long as infection is restricted to the liver, and during the early stages of the erythrocytic cycle. Depending on the malaria species and the health condition of the infected person, this prepatent period (= period without symptoms) can last a number of days or even several months.
Diagnosis
Diagnosis of malaria is usually by examination of thin blood smears and thick blood films. Although live parasites are visible in fresh blood under the microscope, diagnosis relies on stained films and smears which permits not only the recognition of an infection but also the distinction of the different malaria species. This is important, because disease symptoms and prognosis depend on the species.
Plasmodium falciparum, thin blood smear showing trophozoites (ring stages) in the erythrocytes, and gametocytes. Note: some erythrocytes with two trophozoites, and the high proportion of infected erythrocytes. From CDC PHIL 2704
Plasmodium vivax, thin blood smear showing large trophozoite in an erythrocyte. Note the numerous pigment dots in the infected erythrocyte (= Schüffner's dots) characteristic of P. vivax and P.ovale infections. From CDC PHIL 5863
Plasmodium malariae, thin blood smear showing schizont. From CDC PHIL 2715
Plasmodium ovale, thin blood film showing trophozoite (ring stage) in an erythrocyte. Note the fine dots (= Schüffner's dots) in the infected erythrocyte. From CDC PHIL 2716
Symptoms
Most dangerous is Plasmodium falciparum which causes “tropica” and often “cerebral” or “pernicious” malaria, and frequently leads to death. However, other species also cause serious symptoms and sometimes death, although the vast majority of fatal cases are due to P. falciparum.
Symptoms of malaria caused by the various parasites include periodic fever and chill attacks (P. falciparum: every third day or even every day; P. vivax and ovale: every third day = “tertian” malaria; P. malariae: every fourth day = "quartan" malaria). Other symptoms include severe headache, joint pains, nausea, haemoglobin in the urine, and retinal damage. In P. falciparum, blackwater fever (large quantities of haemoglobin in the urine), enlarged spleen and renal failure are not uncommon.
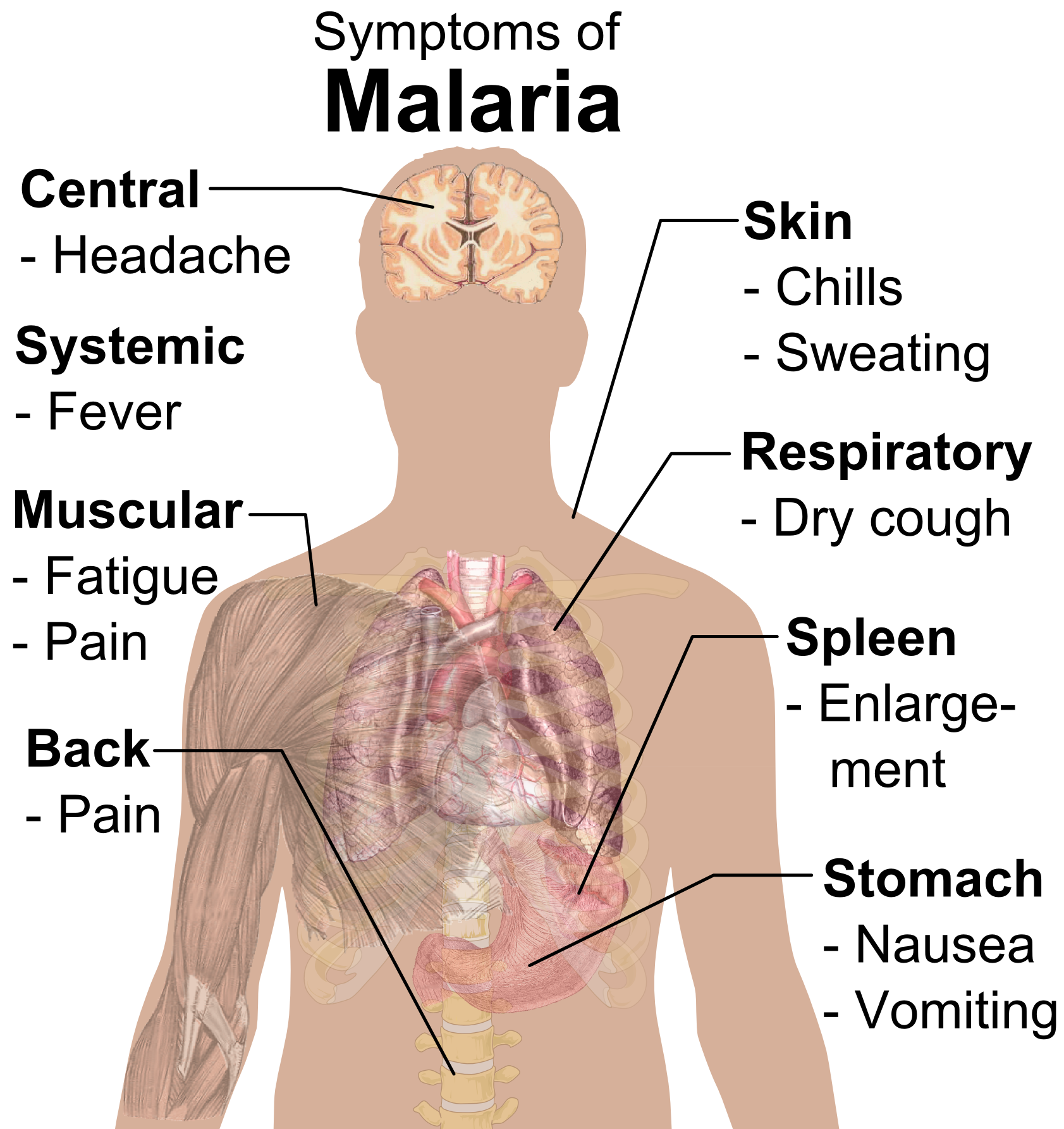 Symptoms may disappear after a while, usually due to treatment, but relapses (= re-occurrences of attacks) are possible, often after many years.
Symptoms may disappear after a while, usually due to treatment, but relapses (= re-occurrences of attacks) are possible, often after many years.
The vector
Vectors of human malaria all belong to the genus Anopheles which require water bodies (stagnant pools, creeks, rivers, small water bodies on epiphytes, etc., depending on the species) for completion of their life cycle. Species of this genus can be distinguished from other mosquito genera by a number of characters, among them the following: eggs with air sacs or floats are laid singly (and not in rafts) in water, larvae lie parallel to the surface film in water when breathing or feeding, breathing tubes of pupae are short and broad at margin, and adults of most species rest with the proboscis in a straight line with the body. - Only female Anopheles feed on blood and transmit malaria; males feed on plant juices.
CDC PHIL ID 7862 Anopheles albimanus, a malaria mosquito feeding on a human.
Experiments have shown that many species of Anopheles can be infected with species of human Plasmodium. However, in nature it is usually a single species or very few that transmit the disease in a particular geographical area. Since each species has different ecological requirements, particularly for breeding, eradication programs for malaria require detailed studies of the ecology of the vectors in each area, which in turn necessitates a detailed knowledge of the morphology and life cycle of the species involved.
CDC PHIL 7949
Anopheles minimus, a malaria vector feeding on a human host
Intra-uterine infections
In some instances, the new-born baby may develop malaria symptoms as the result of infections acquired from the mother as a foetus. Also, very rarely blood transfusions or sharing of needles may transmit the parasite.
Malaria control
The fight against malaria has been fought on several fronts: 1) Prevention of infection by using mosquito nets and insect repellents.2) Mosquito eradication using insecticides (which sometimes have severe environmental impacts), combined with detailed ecological studies to permit the correct application of insecticides. 3) Prophylactic drugs, and drugs used for treatment like quinine, chloroquine, arteminisin, atebrin etc. etc., to which – however – resistance developed and is developing fairly fast, so that new medications have to be developed continually. 4) Development of vaccines. To date, projects to develop vaccines against malaria have been successful only to a marginal degree. No vaccine exists that protects the majority of people against several strains of even one species of malaria.
Geographical distribution of malaria
Human malaria is not restricted to tropical/subtropical regions, but has occurred - in history - at high northern and southern latitudes. For example, is was not rare in the Netherlands and northern Russia, although never as common as at low latitudes. Due to effective eradication programs, it is now almost exclusively found in tropical/subtropical regions.
CDC travel Advisory and Global Malaria Risk Map
Wellcome Trust 2007 Malaria Map
Organizations Involved in Global Eradication of Malaria
Too many players on the same target, yet lack of resources.
Different priorities, agendas resulting in wasted efforts to resolve donor differences
Resources wasted on meetings, travel, glossy printouts and reports
Market success missing as a driving factor
Measurable targets not well defined
Projects with low probability of success rarely terminated
Lessons learned from past failures rarely factored in current campaignsNeed a single United Nations of Malaria
Fig 1 Global Malaria Eradication 2009
![[MMV]](http://www.mmv.org/IMG/logo_top.jpg)













UN Special Envoy for Malaria, UNDP, UNICEF
GSK, WEF, Boston Consulting Group, MacroInternational, Carter Center, DFID
Universities: Columbia, London School of Tropical Medicine, Johns Hopkins, Imperial College, Harvard, Swiss Institute of Tropical Medicine,
Africa Fighting Malaria, Artimisin and Farming international, Fogarty International Center, Global Health Council, Foundation for Innovative New Diagnosis, Innovative Vector Control, Intelligent Insect Control, Development Finance International
The Limits and Intensity of Plasmodium falciparum Transmission: Implications for Malaria Control and Elimination WorldwideCarlos A. Guerra, Priscilla W. Gikandi, Andrew J. Tatem, Abdisalan M. Noor, Dave L. Smith, Simon I. Hay, Robert W. SnowPLoS Med 5(2): e38
Figure 1. P. falciparum Malaria Risk Defined by Annual Parasite Incidence (top), Temperature, and Aridity (bottom)
Areas were defined as stable (dark-red areas, where PfAPI ≥ 0.1 per thousand pa), unstable (pink areas, where PfAPI < 0.1 per thousand pa), or no risk (light grey). The few areas for which no PfAPI data could be obtained, mainly found in India, are coloured in dark grey. The borders of the 87 countries defined as P. falciparum endemic are shown. Highland areas where risk was excluded due to temperature appear in light grey. The aridity mask excluded risk in a step-wise fashion, reflected mainly in the larger extents of unstable (pink) areas compared to the top panel, particularly in the Sahel and southwest Asia (southern Iran and Pakistan).
WHO was the first international body to focus on diseases and health problems of developing countries. The early years were the period of new drug discoveries, antibiotics and the donor nations of the West and WHO. It was assumed that they had the means and drugs in hand to eradicate most of the tropical disease like malaria and TB. Most of these top driven early efforts ordered by donor countries of the West failed. The Rockefeller Foundation had funded and started the first global campaign to eradicate malaria by spraying of insecticide DDT and bednets to keep mosquitos out. The programme was successful in some European countries. The impact in developing countries was mixed. The newly formed WHO promptly adopted the malaria eradication as its top priority in early 1960s. The eradication campaign ran for almost 15 years and was a failure in most developing countries. The concerns about the evironmental impact of widespread use of chlorinated pesticides and the high cost(almost 30% of WHO budget) with poor results led to the demise of the second global malaria eradication project.


By mid 1970s units were formed within WHO to deal with Tropical Disease Research (TDR) , Vaccines and Child and Maternal Health. TDR initiated and started global efforts to eradicate and eliminate malaria, smallpox and tuberculosis without local input or involvement. Lack of funds and the use of low cost drug treatment led once again to failure of such campaigns and development of drug resistance. Although choroquine had become ineffective (drug resistance) it was used until 2001 because it was cheap and alternatives were more expensive. Use of artimisinin was recommended in 2001, it costs more and requires monitoring for cardiotoxicity. Ridley and Fletcher have discussed the history and achievements of TDR and how to deal with future challenges (http://www.who.int/tdr/about/pdf/nature_reviews_micro1899.pdf). TDR timelines of key achievements is available at http://www.who.int/tdr/about/pdf/anniversary_book_timeline.pdf.
The global burden of disease, malaria and TB prevalence and distribution is available at
. WHO Data and map : http://rbm.who.int/wmr2005/html/map1.htm
http://www.who.int/healthinfo/global_burden_disease/GBD_report_2004update_part4.pdf
 Map 2. Global distribution of dominant malaria vectors, 2003 ( 75)
Map 2. Global distribution of dominant malaria vectors, 2003 ( 75)
 http://www.who.int/tb/publications/2009/airborne/background/map.JPG
http://www.who.int/tb/publications/2009/airborne/background/map.JPG
 http://upload.wikimedia.org/wikipedia/commons/0/08/Paludisme_-_Frequence_statistique.png
http://upload.wikimedia.org/wikipedia/commons/0/08/Paludisme_-_Frequence_statistique.png
 The Global Fund to fight AIDS, Tuberculosis and Malaria
The Global Fund to fight AIDS, Tuberculosis and Malaria (
(http://www.theglobalfund.org/en) under UN system in Geneva, has spent millions to provide nets, insecticides and malaria treatments in poor and developing countries.
 The MMV was the first PPP formed in 1999 and since then has developed an R&D portfolio of over 60 projects. MMV was founded by WHO, International Federation of Pharmaceutical Manufacturers Association,
The MMV was the first PPP formed in 1999 and since then has developed an R&D portfolio of over 60 projects. MMV was founded by WHO, International Federation of Pharmaceutical Manufacturers Association, IFPMA,
Global Forum for Health GFHR,
Rockefeller Foundation,
The World Bank,
ABPI,
The Wellcome Trust and Swiss Agency for Development.
 A selection of pages within the site most relevant to the media:
A selection of pages within the site most relevant to the media:
http://www.who.int/mediacentre/factsheets/fs094/en/index.html
17 October 2011 -- An updated WHO factsheet shows that there were 225 million cases of malaria and an estimated 781 000 deaths in 2009, a decrease from 233 million cases and 985 000 deaths in 2000.

MMV at a Glance - a brochure about MMV
Download MMV at a Glance (High Resolution PDF) (4MB)
Download MMV at a Glance (Low Resolution PDF) (1MB)
 MMV en français - guide des pages MMV en français
MMV en français - guide des pages MMV en français
Medical News Today - 24 minutes ago
The huge increase in malaria control programs since 2008 is starting to provide impressive results, says WHO (World Health Organization).
Source: World Health Organization
"World Malaria Report 2010"
 MMV in Natural Products (PDF format, 1.8MB)
June 2009
MMV in Natural Products (PDF format, 1.8MB)
June 2009
The essential reference for malaria control programmes around the world
March 2010
co-authored by Dr Timothy N.C. Wells, Dr Jeremy N. Burrows and Dr J. Kevin Baird
February 2010
Article by Tim Wells in Preclinical Drug Development
September 2011
Interactive science portfolio
MMV’s portfolio focuses on delivering efficacious medicines that are affordable, accessible and appropriate for use in malaria endemic areas. Specifically, the goal is to develop products that will provide: efficacy against drug-resistant strains of Plasmodium falciparum, potential for intermittent treatments (infants and pregnancy), safety in small children (less than 6 months old), safety in pregnancy, efficacy against Plasmodium vivax (including radical cure), efficacy against severe malaria, and transmission-blocking treatment.
The MMV project portfolio is updated quarterly. Click on a project below for more information.
MP - miniportfolio: MMV pioneered the miniportfolio model. The model assures flexibility in allocation of resources – established between MMV and a Pharmaceutical or Biotechnology partner – from one project to another. In this way, resources are utilized as efficiently as possible.
Research
Lead generation
Lead optimization
Translational
Preclinical
Phase I
Phase IIa
Development
Phase IIb/III
Registration
Phase IV

MMV_portfolio
MMV_portfolio
Andrew Witty announces 'open innovation' strategy focusing on neglected diseases and malaria

The development of vaccine for malaria led to the formation of MVI by PATH. Malaria kills over 1 million patients each year and infects over 300-500 million persons resulting in a $12 billion loss of productivity in Afrian countries. The disease can be prevented, diagnosed rapidly and treated by insecticides spays to control mosquitos, bednets to prevent mosquito biting human victims and rapid field test for Plasmodium falciparum infections. The cheap drugs chloroquine/sulphadoxine are not effective due to drug resistance and artimisin based treatment costs much more and requires monitoring. Phase III trials with vaccine show only 30% efficacy (only 1 out of 3 person is protected by the vaccine). such a vaccine if approved can still save over 300,000 deaths each year at relativly low cost and simple treatment. there are 16 malaria vaccines in clinical trials.
First results from ongoing Phase III trial show GSK malaria vaccine candidate, RTS,S* reduces the risk of malaria by half in African children aged 5 to 17 months
Issued: Tuesday 18 October 2011, London UK
Half the world’s population is at risk of malaria which is responsible for close to 800,000 deaths each year, most of whom are children under five in sub-Saharan Africa
First results from a large-scale Phase III trial of RTS,S, published online today in theNew England Journal of Medicine (NEJM), show the malaria vaccine candidate to provide young African children with significant protection against clinical and severe malaria with an acceptable safety and tolerability profile. The results were announced today at the Malaria Forum hosted by the Bill & Melinda Gates Foundation in Seattle, Washington.
NEJM October 18, 2011 | The RTS,S Clinical Trials Partnership
(DOI: 10.1056/NEJMoa1102287)


 At the end of World war II, the disease was endemic all over the world, it has now been eradicated in most of the Western World and over 100 countries. Development of resistance to insecticides, environmental concerns, lack of funds, political will for sustained campaign, high cost of artimisin based treatment regiment and long duration of treatment have derailed several past and current eradication campaigns.
At the end of World war II, the disease was endemic all over the world, it has now been eradicated in most of the Western World and over 100 countries. Development of resistance to insecticides, environmental concerns, lack of funds, political will for sustained campaign, high cost of artimisin based treatment regiment and long duration of treatment have derailed several past and current eradication campaigns.
Malaria Vaccine Initiative (MVI). (http://www.malariavaccine.org)
The price of artemisinin has ranged from $ 150-1200/kg during the past decade with current prices around $ 400/Kg.

For evaluation of and new rapid diagnostic tests
Program for Appropriate Technology in Health http://www.path.org
The main objective is to adopt Western developed technolgy and health solution to local conditions in poor countries and make it suitable for use by local communities.
The Frontlines of Malaria:

Drugs for Neglected Diseases Initiative (www.dndi.org)
The award of the Nobel peace prize to Medicine Sans Frontier (Doctors without borders, MSF) led to the formation of this virtual PPP. The high human cost of the neglected diseases (Trypanosomiasis or sleeping sickness, Leishmaniasis, Chagas Disease and Malaria) is due to lack of new drugs to treat patients.
Médecins Sans Frontières MSF, Institut Pasteur, Indian Council of Medical Research,
Oswaldo Cruz Foundation (FIOCRUZ), TDR, and Health Ministry in Malaysia joined to develop new drugs. There were 21 projects in the R&D portfolio of DNDI.

- http://en.wikipedia.org/wiki/File:Symptoms_of_Malaria.png
New medicines to improve control and contribute to the eradication of malaria
Timothy N. C. Wells, Pedro L. Alonso & Winston E. Gutteridge
Nature Reviews Drug Discovery 8, 879-891 (November 2009) | doi:10.1038/nrd2972
The persistent threat of resistance means that new molecules with novel mechanisms of action are continually required. Furthermore, the recent call for the elimination and eradication of malaria has prompted an extension of the stages of the life cycle of malaria parasites that should be targeted by new molecules. Recent advances in genome-based technologies and in in vitro screening of whole parasites have broadened the range of therapeutic targets and are accelerating the development of a new generation of treatments for both malaria control and eradication.

11 October 2011
With funding from European Union under Framework Seven, a Consortium of 10 organisations, CRIMALDDI*, has developed an integrated and prioritised 5-year Roadmap for antimalarial drug discovery. This is to guide donors, policy makers, and researchers. The Roadmap will be launched today, at the meeting of the All-Party Parliamentary Group on Malaria and Neglected Tropical Diseases (APPMG) in Westminster. It will help to design the European antimalarial research agenda for the next decade as well as contribute to the setting of global priorities.
Famous Victims
Tutankhamun
Alexander the Great
Genghis Khan
King Edward IV
Several Popes
Oliver Cromwell
Lord Byron
Hawass, Z; Gad, ZY; Ismail, S; Khairat, R; Fathalla, D; Hasan, N; Ahmed,A;Elleithy, H; Ball, M; Gaballah, F; Wasef, S; Fateen, M; Amer, H; Gostner, P; Selim, A; Albert Zink, A; Pusch, CM. Ancestry and Pathology in King Tutankhamun's Family. JAMA. 2010;303(7):638-647.
Image from Wikipedia
Malaria Patients
George Washington
Abraham Lincoln
Theodor Roosevelt
John F Keneddy
Christopher Columbus
Jesse James
Lord Nelson
Artur Conan Doyle
Ernest Hemingway
M K Gandhi
Ho Chi Minh
Leon Trotsky
Nobel Prize for Malaria
1902 Ronald Ross
1907 Lavaren
1948 Muller



 Social Bookmarking
Social Bookmarking  Social Bookmarking
Social Bookmarking

















![[MMV]](http://www.mmv.org/IMG/logo_top.jpg)









 MMV at a Glance - a brochure about MMV
MMV at a Glance - a brochure about MMV 
















You made some good points there. I did a search on the topic and found most people will agree with your blog.
RépondreSupprimerMalaria Detection Microscope africa